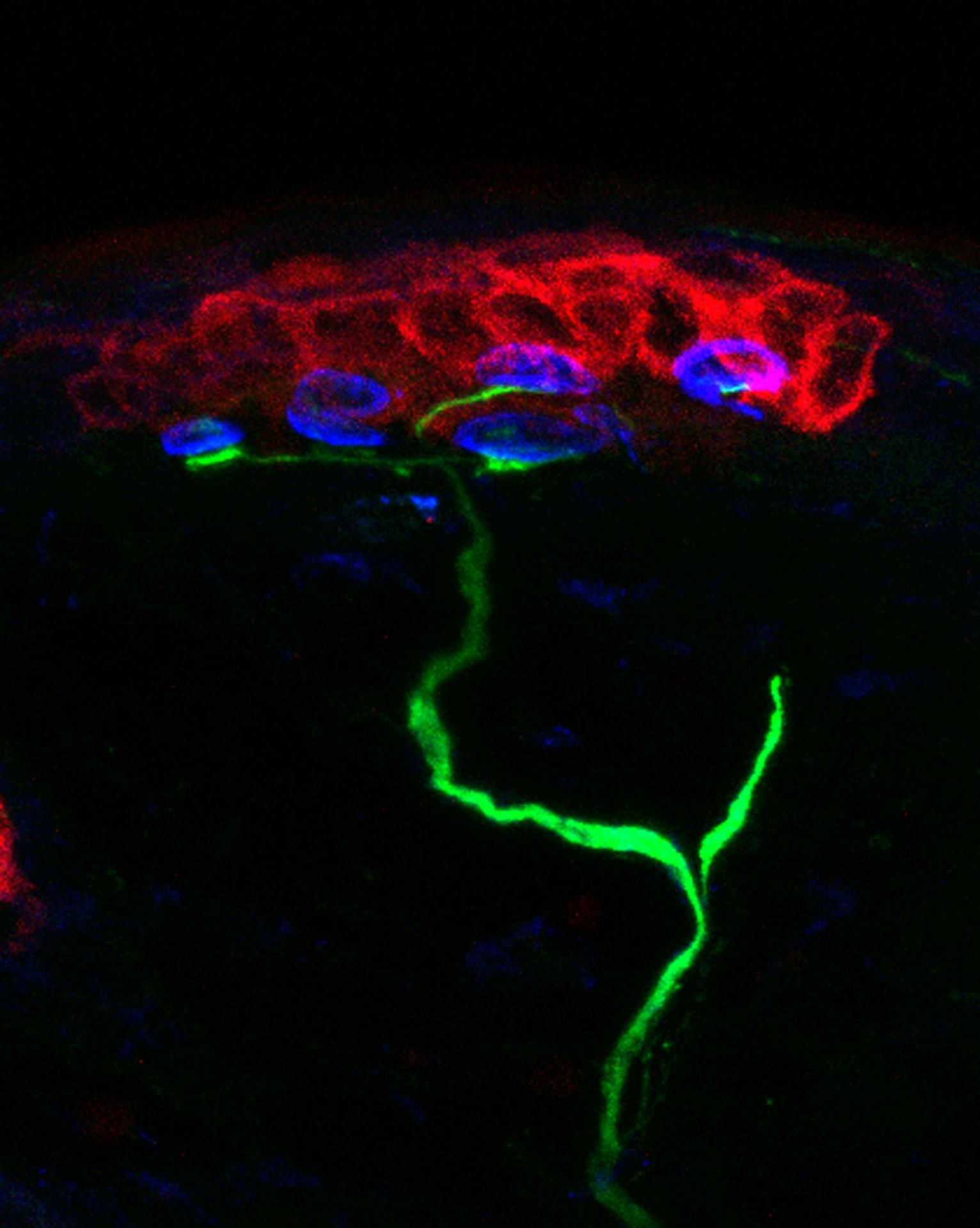
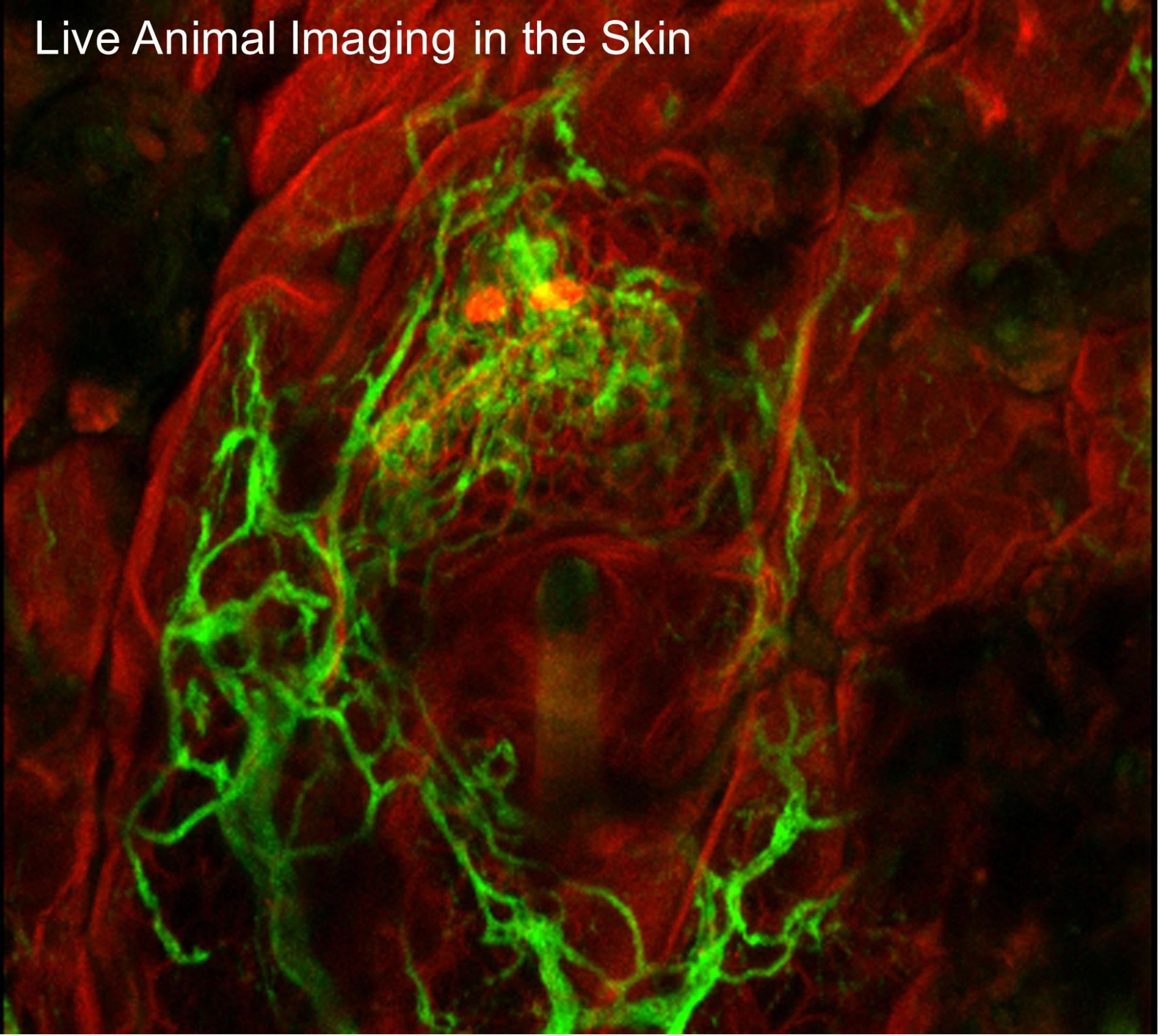
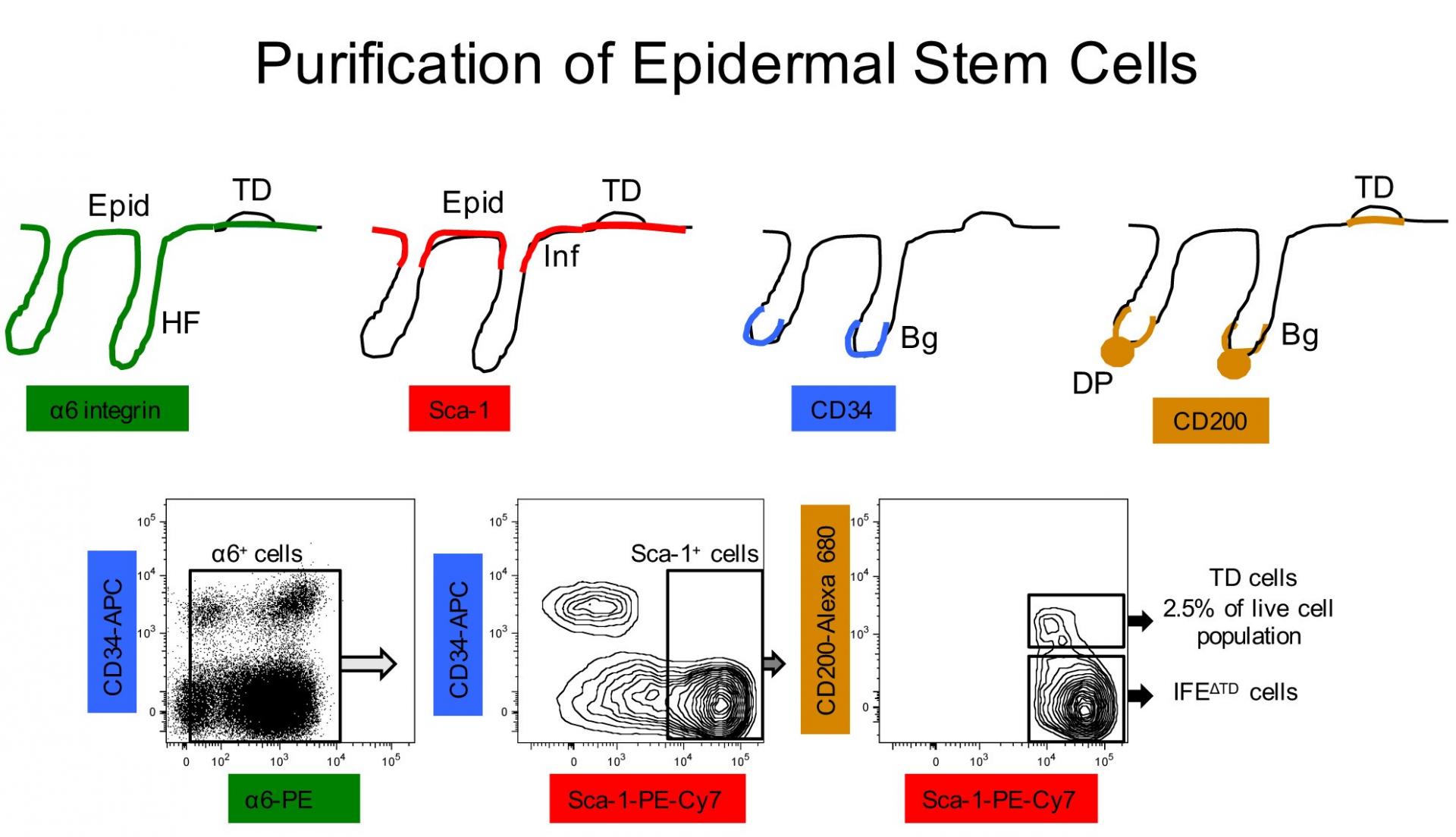
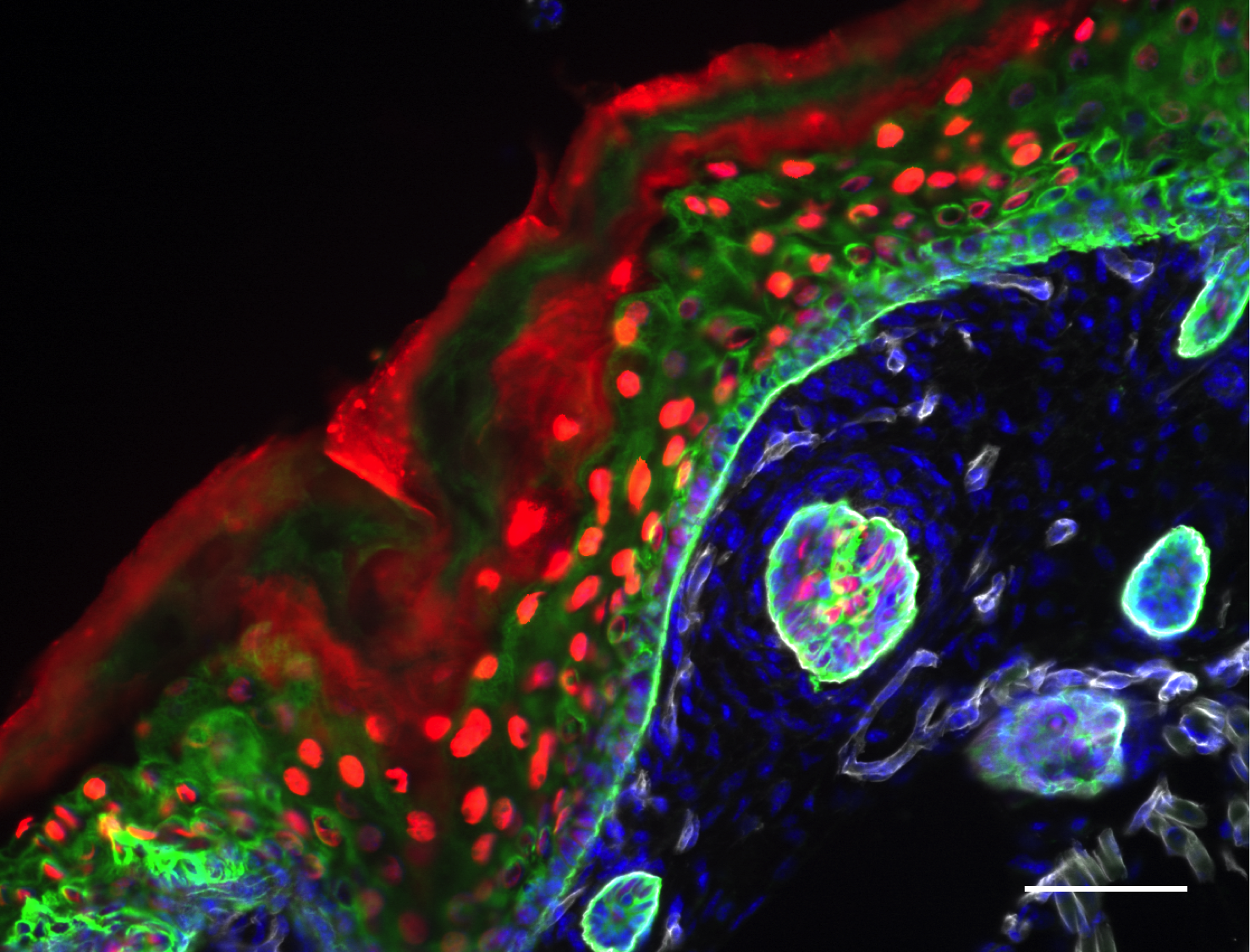
The epiCURE Skin Stem Cell Imaging and Manipulation Core (SCIM) was established to facilitate skin disease research via the propagation and manipulation of various state of the art in vitro and in vivo experimental systems that model skin biology. To assist epiCURE investigators in the phenotypic and mechanistic analyses of skin disease, the SCIM will provide a wide array of histological, immunohistochemical, and molecular-based tools to characterize gross and microscopic morphology, functional features and gene expression in skin of human and genetically engineered laboratory animals.